Decoding the pH Scale: Unveiling the Acidity and Alkalinity Around Us
Have you ever wondered why lemon tastes sour, or why soap feels slippery? The answer lies in a fundamental chemical property called pH. The pH scale is a crucial tool in chemistry, biology, and everyday life, helping us understand whether a substance is acidic, neutral, or alkaline (also known as basic).
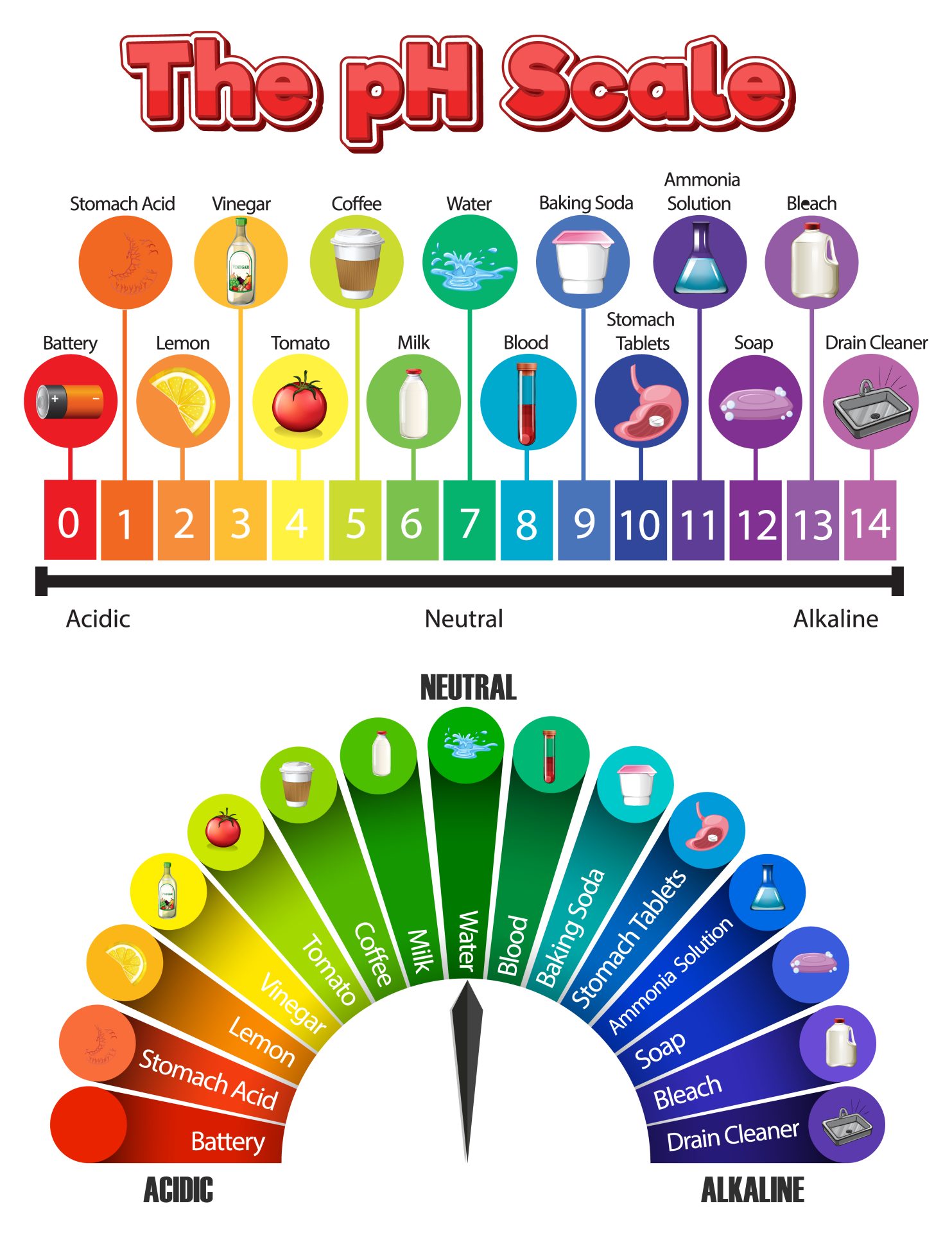
The image above beautifully illustrates the pH scale, showing a range of common substances and their corresponding pH values. Let’s dive into what pH means and why it’s so important.
What Exactly is pH?
The term “pH” stands for “potential of Hydrogen” or “power of Hydrogen.” It’s a numerical scale used to specify the acidity or basicity of an aqueous solution. In simpler terms, it measures the concentration of hydrogen ions (H+) in a solution.
- High concentration of H+ ions: Indicates an acidic solution.
- Low concentration of H+ ions: Indicates an alkaline (basic) solution.
The pH scale typically ranges from 0 to 14.
Understanding the pH Scale: Acidic, Neutral, and Alkaline
As shown in the image, the pH scale is divided into three main categories:
- Acidic (pH 0-6):
- Substances with a pH value less than 7 are considered acidic.
- Acids have a high concentration of hydrogen ions.
- They often taste sour (like lemon or vinegar) and can be corrosive.
- Examples from the image: Battery acid (0-1), Stomach Acid (1-2), Lemon (2-3), Vinegar (2-3), Coffee (4-5), Tomato (4-5).
- You’ll notice that the lower the pH number, the stronger the acid. For instance, battery acid at pH 0-1 is extremely corrosive, while coffee at pH 4-5 is a much weaker acid.
- Neutral (pH 7):
- A pH value of exactly 7 indicates a neutral substance.
- Neutral solutions have a balanced concentration of hydrogen and hydroxide (OH-) ions.
- Example from the image: Pure Water (7). Our bodies strive to maintain a neutral or slightly alkaline pH for many biological processes.
- Alkaline / Basic (pH 8-14):
- Substances with a pH value greater than 7 are considered alkaline or basic.
- Bases have a lower concentration of hydrogen ions and a higher concentration of hydroxide ions.
- They often feel slippery (like soap or bleach) and can also be corrosive, especially strong bases.
- Examples from the image: Blood (7-8), Milk (6-7), Baking Soda (8-9), Stomach Tablets (9-10), Ammonia Solution (11-12), Soap (11-12), Bleach (12-13), Drain Cleaner (13-14).
- The higher the pH number, the stronger the base. Drain cleaner at pH 13-14 is a very strong base and highly corrosive.
Why is pH Important?
The pH of a substance influences countless processes and applications:
- Biology and Health: Our bodies maintain a very precise pH range in different organs. For instance, our blood’s pH is tightly regulated around 7.35-7.45. Stomach acid, on the other hand, is highly acidic (pH 1-2) to break down food.
- Agriculture: Soil pH directly affects nutrient availability for plants. Farmers often adjust soil pH to optimize crop growth.
- Environmental Science: The pH of lakes and rivers is crucial for aquatic life. Acid rain, for example, can drastically lower pH, harming ecosystems.
- Everyday Products: From shampoos (often slightly acidic to match hair’s pH) to cleaning products (many are alkaline to cut grease), pH is carefully controlled.
- Industrial Processes: Many chemical reactions and manufacturing processes are highly sensitive to pH levels.
Measuring pH
pH is typically measured using:
- Litmus Paper or pH Indicator Strips: These strips change color when dipped into a solution, and the color is then matched to a chart to determine the pH.
- pH Meters: These electronic devices provide a more precise digital reading of the pH.
- Liquid Indicators: Specific chemical solutions that change color at different pH ranges.
The pH scale is more than just a chemical concept; it’s a fundamental aspect of the world around us. By understanding whether a substance is acidic, neutral, or alkaline, we gain valuable insights into its properties, its impact on living systems, and its role in various applications. So, the next time you sip your coffee or use soap, you’ll know there’s a fascinating world of pH at play!
